Science Lesson: Discovering Chemical Reactions
In this lesson, students conduct an experiment that explores how the amount of energy transferred into an endothermic reaction changes when the mass of a reactant changes. Because matter cannot change without enough energy, all chemical reactions involve the transfer of energy between the reaction and the environment. Depending on the energy required in the chemical reaction, energy is either transferred from the chemical reaction to the environment or from the environment to the chemical reaction.
Science Big Ideas
- Energy transfers into or out of chemical reactions in a similar way to how energy transfers in energy systems.
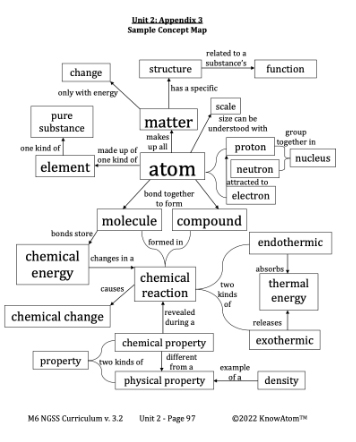
- Every chemical reaction needs energy to get started. This initial input of energy is called activation energy. Once the reaction begins, some reactions absorb more energy from the environment than they release, while others release more energy into the environment than they absorb.
- When a reaction is exothermic, energy is released into the environment. It is transferred from the reaction to the environment.
- When a reaction is endothermic, energy is absorbed from the environment. Energy is transferred from the environment into the reaction.

Discover Complete Hands-on Screens-off Core Science Curriculum for K-8 Classrooms
Prepared hands-on materials, full year grade-specific curriculum, and personalized live professional development designed to support mastery of current state science standards.
Science Essential Questions
- How can energy transfer within an energy system when two objects collide?
- How is energy transferred in a chemical reaction?
- How would you compare endothermic reactions with those that are exothermic?
- What evidence can be used to show when a reaction is exothermic or endothermic?
- How is the mass of the reactants related to the mass of the products in both endothermic and exothermic reactions?
Common Science Misconceptions
Misconception: Cold/frozen substances do not have any thermal energy.
Fact: All matter has thermal energy. The atoms or molecules that make up a substance are always moving because of thermal energy.
Misconception: In a chemical reaction, the atoms of the reactants are transformed into other kinds of atoms.
Fact: The number and kinds of atoms in the reactants do not change in a chemical reaction.
Science Vocabulary
Endothermic : a process that absorbs energy from the environment
Exothermic : a process that releases energy into the environment
Lexile(R) Certified Non-Fiction Science Reading (Excerpt)
A Professional Glassblower
Kiva Ford spends his days making complex glass instruments that scientists use in the laboratory. When he comes home from work, he focuses on artistic glass structures. He designs goblets, flower vases, and intricate animal sculptures. For professionals like Kiva, glassblowing combines art and science. To create scientific instruments and elegant glassware, glassblowers need to have a deep understanding of what glass is, how it is formed, and its molecular structure.
Kiva’s interest was sparked in part by one property in particular: glass’s state of matter. “My experience with glass was that it’s solid,” Kiva said in an interview with the e-commerce website Etsy. “And then when I was introduced to glassblowing, all of a sudden it’s not a solid anymore. It’s a liquid. It almost feels like honey in between your hands.”
Energy Transfer: Exothermic Processes
Glassblowers all work with torches. A torch is a tool that produces controlled fire at the end of it. The torch produces the extreme temperatures needed to shape and form the particular shapes needed in glassblowing. Torches work because of a chemical reaction.
In any chemical reaction, the reactants and products form a system. The environment is everything else, including the air or any substance mixed with the reactants. As the reactants combine and rearrange, energy is exchanged between the system and the environment.
Every chemical reaction needs energy to get started. This initial input of energy is called activation energy. For example, when someone strikes a match to light a candle, they provide the activation energy needed to start a fire, which is a chemical reaction.
Every chemical reaction needs energy to get started. This initial input of energy is called activation energy. For example, when someone strikes a match to light a candle, they provide the activation energy needed to start a fire, which is a chemical reaction.
Once the reaction begins, some reactions absorb more energy from the environment than they release. Others release more energy into the environment than they absorb. Any process in which the system loses heat to the environment is called exothermic. “Exo-” means to give off. Because the energy is released as heat, the environment’s temperature will increase. The environment’s temperature increases because it means that the reaction has released thermal energy into the environment.
Endothermic Processes
Whenever a process occurs in which the system absorbs heat, it is called endothermic. “Endo-” means to draw in. In an endothermic reaction, the environment’s temperature decreases. This is because the reaction has absorbed energy from the environment.
A chemical ice pack uses an endothermic reaction to get cold. Many ice packs have ammonium nitrate and water kept in separate sections with a thin barrier between them. When you break the barrier, the water and ammonium nitrate combine. They absorb energy from the environment. This is why the ice packs get cold. When the reactants have fully reacted, the chemical reaction stops.
The strength of a chemical reaction can be measured by the amount of energy absorbed or released by the reaction. When more reactants are combined in a chemical reaction, the amount of energy that is absorbed or released increases.



Hands-on Science Activity
In this lesson, students use the scientific process to analyze the phenomena of endothermic chemical reactions, specifically how increasing one of the reactants affects the magnitude of the temperature change of the reaction. Students record the temperature of their chemical reaction experiments over a set amount of time and calculate the temperature change for each chemical reaction. Students then graph their data to help them identify patterns in how thermal energy is transferred in an endothermic chemical reaction.
Science Assessments
KnowAtom incorporates formative and summative assessments designed to make students thinking visible for deeper student-centered learning.
- Vocabulary Check
- Lab Checkpoints
- Concept Check Assessment
- Concept Map Assessment
- And More...

See How KnowAtom Aligns to NGSS Science Standards
Discover hands-on screens-off core science curriculum for student centered K-8 classrooms. KnowAtom supports classrooms with all hands-on materials, curriculum, and professional development to support mastery of the standards.

