Science Lesson: Understanding Ocean Salinity and Density
In this lesson, students apply what they’ve learned about the variations in the water cycle to explore how evaporation affects ocean water density, which in turn affects weather and climate.
Science Big Ideas
- Understanding the ocean’s salinity (the measure of all the salts dissolved in water) and how that salinity differs around the world and changes over time is essential for scientists who want to understand the movement of water around the planet.
- Ocean water is made up of different kinds of matter.
- Salt water is a mixture, which means it is made up of two or more pure substances that are mixed together but not chemically bonded.
- Variations in the amount of water that cycles around the planet, caused by Earth’s tilt and orbit, play a significant role in the differing amounts of salt in the world’s oceans.

Discover Complete Hands-on Screens-off Core Science Curriculum for K-8 Classrooms
Prepared hands-on materials, full year grade-specific curriculum, and personalized live professional development designed to support mastery of current state science standards.
Science Essential Questions
- How does the hydrosphere and the atmosphere interact with the geosphere to cause the oceans to become salty?
- Why is there so much salty ocean water on Earth?
- What evidence supports the claim that salt water is a mixture?
- How can thermal energy separate a mixture of salt and water?
- Why is water vapor always fresh water?
- Why does the freezing of seawater cause the ocean to become saltier?
- Why does rain and other forms of precipitation cause ocean water to become less salty?
- How does temperature affect the density of water?
- How does ocean depth affect density?
- Why do scientists care about ocean salinity, temperature, and therefore density?
Common Science Misconceptions
Misconception: Oceans have the same salinity everywhere.
Fact: Ocean salinity can vary by location and season.
Science Vocabulary
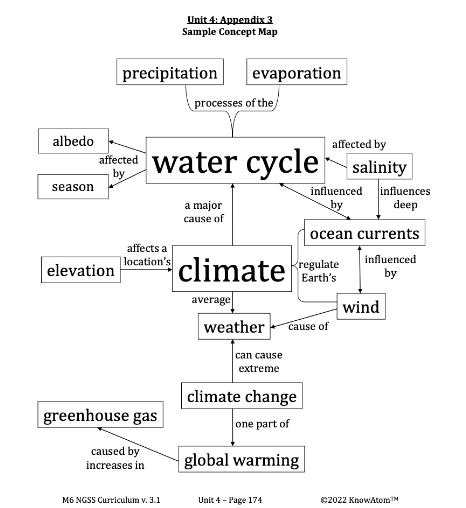
Climate : the average weather in a location over 30 years or more
Evaporation : the process of liquid water changing into water vapor, its gas state
Ocean Currents : paths of flowing ocean water that push warm and cold water to different parts of the planet
Precipitation : the process of water falling back to Earth in the form of rain, snow, sleet, or hail
Salinity : the measure of all the salts dissolved in water
Season : a period of time characterized by specific weather patterns and by the length of day and night
Water Cycle : the circulation of water through the hydrosphere from Earth’s surface to the atmosphere and back
Weather : the conditions of the atmosphere (temperature, humidity, wind speed, and precipitation) at a particular place and time
Lexile(R) Certified Non-Fiction Science Reading (Excerpt)
Studying the Ocean’s Saltiness
Since 2011, a satellite called Aquarius has been orbiting Earth from 644 kilometers (400 miles) above the surface. Its mission has been to gather data about the salinity in the surface seawater. Salinity is the measure of all the salts dissolved in water. If you’ve ever gone swimming in the ocean and accidently swallowed some water, you’ve tasted how salty the ocean is.
For hundreds of years, scientists physically measured the salinity of different parts of the ocean from ships and buoys. This provided limited data, however. Some parts of the ocean were much harder for scientists to reach and measure than others. In response to this problem, a team of about 100 scientists and engineers developed and launched the Aquarius satellite in 2011. The Aquarius satellite improves on older methods of gathering data because it continuously monitors and collects data on ocean salinity around the planet.
Understanding the ocean’s salinity, including how that salinity differs around the world and changes over time, is essential for scientists who want to understand the movement of water around the planet. This in turn has major implications for weather and climate. Weather is the conditions of the atmosphere (temperature, humidity, wind speed, and precipitation) at a particular place and time. Climate is the average weather in a location over 30 years or more.
How Oceans Get Salty
Understanding salinity first begins with an understanding of how oceans become salty. Oceans become salty because of interactions among different Earth systems. Remember that a system is a set of connected, interacting parts that form a more complex whole. Systems have inputs and outputs. Inputs are what are received by the system. Outputs are what are sent from the system.
Earth has four primary systems that are interconnected, constantly interacting with and influencing one another—the hydrosphere, atmosphere, biosphere, and geosphere.
Earth’s systems are interconnected, constantly interacting with and influencing one another. For example, all of the water on Earth forms an Earth system called the hydrosphere. The hydrosphere includes all of the ice, liquid water, and water vapor on Earth. It is constantly interacting with another Earth system called the atmosphere. The atmosphere is the mixture of gasses, dust, water vapor, and other molecules above Earth’s crust.
One of the gasses in the atmosphere is carbon dioxide. When water falls to Earth’s surface as rain, it carries some of this carbon dioxide, making the water slightly acidic. This slightly acidic water breaks down the rocks on Earth’s surface into smaller particles, including salt and other minerals. This is an interaction between the hydrosphere and the geosphere—the Earth system made up of Earth’s landforms, including mountains, valleys, soil, and sediment.
All water that collects on Earth’s surface will eventually flow downhill to the oceans because of the pull of Earth’s gravity. As water flows downhill, it carries with it the particles of salt and other matter. All water eventually ends up in the ocean, where it deposits the salt and other matter. Over millions of years, this salt has built up in the oceans, creating the salt water of the oceans.
Why Salinity Matters
Ray Schmitt is the lead scientist on a scientific mission that is using data from the Aquarius satellite. Schmitt has long been fascinated by salt in the ocean. He was one of the first scientists to recognize how important the ocean is in the water cycle.
In 2012, Schmitt and a team of scientists set sail for the world’s saltiest patch of open ocean water, located halfway between the Bahamas and the western coast of North Africa. Scientists say this spot is similar to a desert on land, where there is little rainfall and a lot of evaporation. Data suggest that this spot of ocean is actually becoming saltier over time.
The scientists on board the ship stayed for three weeks, taking measurements on the ocean’s salinity, temperature, and other factors. They used many different kinds of scientific tools, including floats, gliders, drifters, moorings, ships, satellites, and computer models. One of the reasons that scientists care about variations in ocean salinity is that they play a major role in deep ocean currents. Ocean currents are paths of flowing ocean water that push warm and cold water to different parts of the planet.
Cold, dense water in the oceans sinks deep and spreads out all around the world. The sinking water is replaced by the warm, less-dense water near the surface that moves to the north. Scientists call this the Great Ocean Conveyor Belt.



Hands-on Science Activity
For the hands-on activity, students carry out an experiment to explore the effect of evaporative water loss on ocean density. Students calculate the density of salt water in an open system and a closed system. They then expose the open condition to evaporative wind over a few days, and compare the change in density that occurs in both systems. Students use the data from the experiment to analyze the relationship between the amount of water evaporation and the density of the water.
Science Assessments
KnowAtom incorporates formative and summative assessments designed to make students thinking visible for deeper student-centered learning.
- Vocabulary Check
- Lab Checkpoints
- Concept Check Assessment
- Concept Map Assessment
- And More...

See How KnowAtom Aligns to NGSS Science Standards
Discover hands-on screens-off core science curriculum for student centered K-8 classrooms. KnowAtom supports classrooms with all hands-on materials, curriculum, and professional development to support mastery of the standards.

